This is important because, unless hydrogen can be made cheaply and in plentiful supply, the fuel cell hydrogen car will face the same roadblocks as the electric car faces with costly batteries and poor range.
At this stage Australia is pretty much off the fuel-cell radar, but hydrogen cars from Hyundai and Toyota – the only two car makers with a car on the market – are on sale or lease in some major markets.
The Hyundai ix35 FCEV, the world’s first mass-produced hydrogen car, is available for lease in Europe, Korea and California with a new generation version on the way. The Toyota Mirai, the first hydrogen car offered for sale at an equivalent price of $110,000, is available in the US, Europe and Japan. Honda is working on a successor to its lease-only Clarity that had 45 examples in the US and Japan from 2008 to 2014.
These cars require hydrogen which is run through a fuel-cell stack where the reaction makes electricity. So the fuel cell car is an EV which makes its power from onboard hydrogen as it drives.

But help on the hydrogen production side looks to be at hand.
Two recent developments, both in the United States, claim to have discovered continuous hydrogen production processes that address the severe problems of the high energy costs, as well as the prohibitive cost of the precious metals associated with, splitting hydrogen from water.
Splitting water has always been see as the holy grail because it has cleaner credentials. But it requires a large supply of electricity to provide hydrogen in sufficient quantities to run cars in any significant numbers. In addition, the metals used in the process -, typically platinum and iridium – are very expensive and rare.
There has been a geopolitical concern that if hydrogen was to be made in large quantities to meet fuel cell car demand by using these metals potentially from nuclear-generated electricity, the world would move from countries beholden to those with oil to countries beholden to those with these precious metals.
Typically today, the majority of hydrogen on the market is, in general terms, made by boiling natural gas or methane. This is energy-intensive and undoes the hoped-for clean credentials of hydrogen. Additionally, the capacity of manufacturers to increase production of hydrogen is limited.
But a team at Stanford University has developed a continuous hydrogen production process that will run 24 hours a day, seven days a week by looking at a branch of lithium ion battery technology to arrive at a low-cost, low-energy solution to splitting hydrogen from water.

Hongjie Dai, Professor of Chemistry
The team have been making hydrogen and oxygen continuously for up to 200 hours using a 1.5 volt AAA battery with an efficiency of 82 per cent at room temperature.
What is exciting at this early stage is that the Stanford team are suggesting that the simplicity and low energy production of hydrogen from water in their discovery may make it possible to produce hydrogen for the fuel cell stack “on-the-fly” from water stored in fuel cell cars.
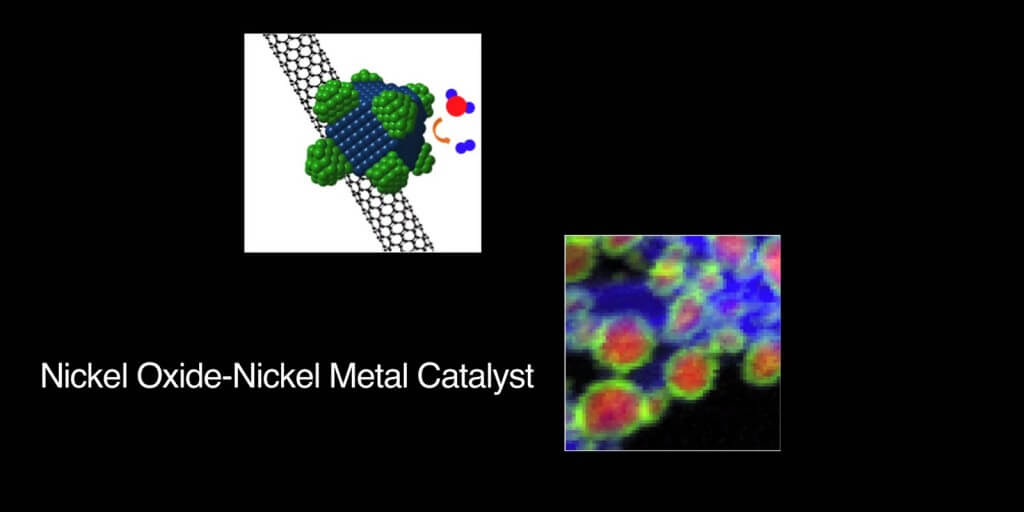
The key to the discovery is a catalyst made of just nickel and iron which are relatively cheap. But the secret is that it is composed of miniscule nanoparticles (smaller that a human hair) that create millions of micro-channels that significantly increase the surface area of the catalyst.
This makes the process eminently more efficient. As a bonus they have found they do not need a second catalyst of a different material which was typically the case until now.
This will significant lower the capital investment and running costs of any scaled up production plant.
The team believes that hydrogen’s ultimate green credentials will come if their process is powered by renewable electricity.

The second development came at Indiana University where scientists have developed a fermentation process that creates hydrogen from water through a biological reaction.
Scientists embedded a hydrogen-producing enzyme into bacteria that acts in a similar way to the hydrogen-forming ability of platinum when used in a fuel cell.
The beauty is that the bacteria can be easily reproduced, can operate at room temperature, is inexpensive and unlike platinum, is not the result of mining and intense manufacturing processes.
The breakthrough came when the scientists manipulated a substance that previously, despite great promise in the process, broke down at temperatures above room temperature and could be readily attacked by chemical compounds in our everyday environment.
The Indiana University team believes the process they developed is now robust enough to use in manufacturing and in cars.
By John Mellor












 Read More: Related articles
Read More: Related articles

